Radioactive Decay Occurs When Atoms of an Unstable Element
6 What everyday contains radiation. Occurs when elements atomic is greater than 83.
Discovery Of Radioactive Decay Zoefact
11 Why are all new elements radioactive.
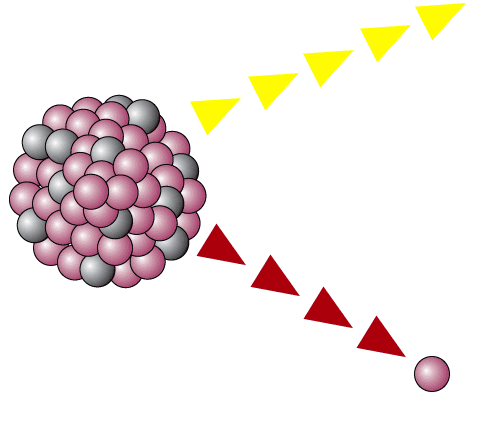
. Join with atoms of another element. 8 How do I tell if an element is radioactive. It results in parent elements to TRANSMUTE daughter elements which is alpha beta gamma.
All elements are radioactive. 10 What is radioactive in simple words. At one half-life half of the original unstable element has decayed.
9 Which type of particle can be emitted by an unstable nucleus quizlet. 11 What is radioactivity in simple terms. TOC Radioactive Decay Lab Introduction.
In the case of radioactive decay instability occurs when there is an imbalance in the number of protons and neutrons in the atomic nucleus. Become part of a fossil B. 5 What radioactive means.
14 Why do atoms want to be stable. Become part of a fossil C. 16 What is the difference between radiation and radioactive atoms.
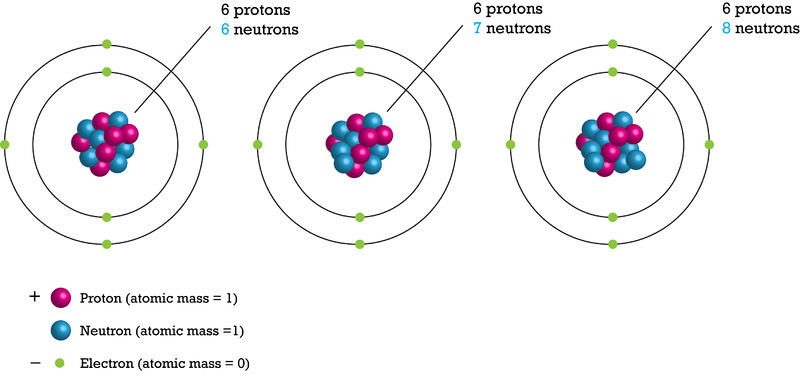
During radioactive decay the unstable nuclei of isotopes emit particles release energy to become stable isotopes After radioactive decay the element changes into. Some isotopes are unstable. Isotopes are atoms of the same element but with different atomic masses.
5 Which type of nuclear decay produces energy instead of a particle. When this occurs a new atom and element are formed. 9 Why is C 14 unstable.
Unstable atoms release particles. 13 Is uranium a radioactive element. 15 Why do some atoms decay.
The half-life for the radioactive decay of U-238 is 45 billion years and is. Break down to form atoms of another element D. Radioactive decay occurs when an unstable atomic nucleus loses energy by emitting energy in the form of emitted particles or electromagnetic waves called radiation.
12 What is radioactive decay in simple terms. 10 How do you make something radioactive. 9 What is the most radioactive place on earth.
An excess of neutrons and protons can cause this instability which leads to the emission of alpha particles beta particles or high-energy photons gamma radiation. Radioactive decay occurs when atoms of an unstable element. 10 What must occur before a radioactive atom stops undergoing further radioactive decay.
Are exposed to chemical weathering D. Are exposed to chemical weathering. Become part of a fossil C.
Isotopes are atoms of the same element thereby having the same number of protons which differ in the number of neutrons in their nucleus. Why do isotopes decay. Every atom seeks to be as stable as possible.
Radioactive decay is a random process by which unstable atoms with an excess of particles andor energy emit radiation to achieve stability. 8 What is an example of radioactivity. Radioactive decay occurs when atoms of an unstable element.
Break down to form atoms of another element B. 3 What causes an element to go through radioactive decay. Break down to form atoms of another element B.
Radioactive decay occurs when atoms of an unstable element. If the nucleus of an atom is unstable eventually it will break apart to lose at least some of the particles that make it unstable. Radioactive decay occurs when an unstable atomic nucleus emits particles or light waves.
7 What is the difference between radiation and radioactive atoms. Join with atoms of another element. 12 Can any element be radioactive.
When the atoms of an element have extra neutrons or protons it creates extra energy in the nucleus and causes the atom to become unbalanced or unstable. Radioactive decay is the rate at which new atoms form. Radioactive decay occurs when an unstable atomic nucleus loses energy by emitting energy in the form of emitted particles or electromagnetic waves called radiation.
The unstable element isotope really will break down to form another kind of atomThe unstable element. Join with atoms of another element C. This occurs because different isotopes have different numbers of neutrons.
4 Why do atoms become radioactive. Occurs in nucleus which is protons and neutrons. The unstable nucleus of radioactive atoms emit radiation.
7 What causes radioactivity quizlet. Radioactive decay occurs when atoms of an unstable element break down to form what. 7 What is an electron emitted by an unstable nucleus.
Isotopes are atoms of the same element. 6 What do radioactive atoms do. Most elements have atoms that come in two or more forms called isotopes.
17 Why does. Radioactive decay occurs when atoms of bartleby. 3 on a question.
Whether radioactive elements can become stable and if so how. During radioactive decay atoms break down releasing particles or energy. A half-life is half of the lifetime of a radioactive element.
5 Which element is most radioactive. 8 What is the process for emitting excess energy from the nucleus of an atom called. Are exposed to chemical weathering D.
Isotopes are atoms of the same element thereby having the same number of protons which differ in the number of neutrons in their nucleus. For example hydrogen has three isotopes that are listed in the table to the right. The rate of decay of a.
6 When an unstable atomic nucleus emits. The radioactive decay of an element occurs at a constant rate. Some isotopes of a given element are more unstable than others causing a nuclear reaction which releases energy to achieve a more.
11 Can you touch polonium.

Notes 11 3 Radioactive Decay Half Lives Ppt Download
0 Response to "Radioactive Decay Occurs When Atoms of an Unstable Element"
Post a Comment